Garden Soil
You’ll know as well as me, when you’re gardening you spend most of your time with your hands in the soil. Whether it be weeding, digging over, or changing up your planting designs it all starts with soil. As gardeners in North Yorkshire with over 50 years experience, we’re here to help you get the best out of your garden soils by dishing the dirt on important matters like your garden soil profile, types of soil, soil improver, soil care and anything else we can think of. Also, if you have any questions or can think of anything that we should write about please get in touch
Why is soil important?… well there’s the obvious, every plant, tree, flora and fungi that is growing today, is growing in soil. It provides them with food, water and anchors plants upright and in doing so it provides the ecological services that are crucial for all life. Soil is also a massive carbon sink and contains 2500 gigatons of carbon, 3 times more than in the atmosphere and 4 times that which is stored in all the plants and animals on the planet. Soil also provides habitats for billions of living organisms. In fact a handful of soil contains more living organisms than there are people on the earth.
Are your plants looking a bit tired and unhappy.
It might because the nutrients in their soil
What garden soil is made of
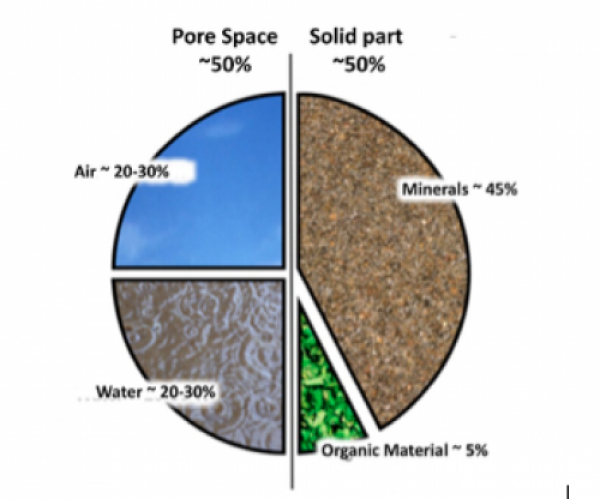
So we now know that soil is full of living things, and is essential to life on this planet. But what is that brown stuff that gets under our finger nails, sticks to our boots and the dog likes to roll in (or the kids if you’re me?). In simple terms, the composition of soil is made up of two halves.
The Solid Bit - which is mostly inorganic materials or ‘rock’, and about 5% organic matter or ‘humus’, (not hummus, which is the delicious dippy chickpea delight I like to gorge myself on …… mmmmmm) and,
The Pore Spaces - of which about 25% is water and 25% is air
How is soil formed?
The type of soil in your garden beds depends on the type of parent rock making up the soil. Obviously the rock present in your soil is broken into tiny fragments by natural processes called weathering. At the point at which the rock fragments are small enough to support plant life - we call it soil.
There are 3 main types of parent rock which make up most soils.
Igneous rock
- these are the oldest rocks resulting from the formation of the earths’ crust. This is rock which was once molten (think lava from a volcano) and then cooled. As it cooled the molten rock formed mineral crystals and resulted in a very hard rock which is slow to weather. Examples of these are granite, basalt and obsidian. The soil formed out of igneous rock has a very high clay content and is rich in the nutrients necessary for plant life.
Sedimentary rock
This rock is made up of accumulations of sediments formed from the weathering of older rocks. These sediments have been washed or blown down to valleys, rivers and out to sea, where they consolidate under pressure to form new rocks. Examples of this are limestone, sandstone or shale.
Metamorphic rock
These are rocks which are formed from both igneous and sedimentary rocks. They have been modified due to the application of great heat and pressure, which causes changes to the texture, mineral content and chemical composition of the rock. Some examples of this are shale turning to slate and limestone turning to marble.
What is weathering?
and how does it happen?
Well folks, it’s all in the name! Weathering is the effect the weather has on rock. But weathering is not just caused by weather. There are other, less obvious processes at play. And these processes can result in some pretty funky and awe inspiring rock formations, some of which people travel a long way to see. Check out our travel blog for more information if geology rocks you!!
So, the three types of weathering, Physical, Chemical and Biological.
Physical Weathering
Glyber Fach - Snowdonia
…doesn’t change the character of the minerals present in the rock, it simply breaks it down into smaller and smaller fragments. It can occur due to temperature changes, such as heating and cooling in deserts or the freeze thaw action which formed these rocks in Snowdonia. It may be the action of rocks tumbling along in a river or the roots of a plant growing in a crack and forcing the gap to widen. Basically any weathering caused by the geological processes of the earth or the environment is called physical weathering.
Chemical Weathering
Limestone Pavement - Malham
…is the decomposition of the original rock by chemicals found in rainwater. These can be broken into three processes.
Carbonation - the process of acidic rainwater dissolving calcium carbonate in limestone, it is this process which has helped the formation of limestone pavements like the one at Malham, Yorkshire pictured above.
Hydrolysis - the chemical breakdown of a rock when combined with water, such as the breakdown of feldspar crystals present in granite. The feldspar turns to clay when it reacts with water, weakening the rock.
Oxidation - the reaction of oxygen with iron present in rock causing it to rust and weaken the rock. When this iron leaches from the rock in to rivers it can cause those rivers to turn a rusty red colour. You can see this in rivers in the Yorkshire Dales.
Biological Weathering
North Yorkshire, Fountains Abbey - Seven Bridges Valley
…is the weakening and eventual breakdown of rocks by plants, animals and microbes. This may be as simple as a plant rooting into a crack in a rock. As the root grows, it exerts pressure on the rock causing it to fracture. Or a burrowing animal moving rocks to the surface, where they become subject to chemical and physical weathering.
A more complex example of biological weathering is that of Lichen. Lichen is a fungi and algae living together in a symbiotic relationship. The fungi releases a chemical which causes rock minerals to break down. The algae in turn feeds off the minerals. This process eventually leads to holes and gaps forming in the rock, leaving it open to further processes of chemical and physical weathering.
Formation
How soil is formed.
So we know the type of soil in your garden depends on the type of parent rock it is made up from, and we know how the parent rock is weathered into smaller and smaller fragments to become part of your soil. But how is the soil itself formed and how did it end up in your garden?
Well, the parent material is not always sedentary. It moves around the earth carried by wind, water, ice or gravity. Soil types can be defined by the way they have been transported and deposited. The three main ways parent materials are transported are:-
By water - in a river or stream the parent materials are sorted into different particle sizes as they are carried along by the water. Large heavier particles are deposited first and are often found close to a water source. Smaller, lighter particles such as clay and silt can be carried long distances and tend to be found farther away in river bed or flood plains. These fine soils are called alluvial soils. They tend to be silt, clay, sand or gravel plus lots of organic matter. These are great soils for growing plants, as they are very fertile. Good examples of alluvial soil deposits can be found in the Mississippi Delta, or along the Nile or Ganges Rivers.
By Wind - similarly to water, wind sorts parent material by particle size. Heavy particles like sand will be blown across short distances, clays form larger particles do the same. Fine particles like silt will be carried long distances, suspended in the wind. Soils made up of these deposits are called loess, and they tend to bury large areas of other soils and rock. Loess is dry and powdery and subject to erosion. Perhaps the best example of this is the Loess or Huangtu Plateau which covers 640,000 km2 of land in Northwest China and has one of the worlds highest erosion rates.
Sedentary - this is soil that has remained in the same place and lies above its parent rock. It can be found all over the world but the largest areas are found in tropical regions such as Brazil, South India and the Philippines.
Soil Profile
Eventually the particles of parent material settle and begin to form the layers which make up soil as we know it. These layers are called horizons and it is these horizons which define soil characteristics. There are 5 main horizons, all laying approximately parallel to the surface of the land, each one differing in their chemical and physical properties and in composition see below.
Soil profile showing horizons
< O - organic matter and undecomposed leaf litters
< A - top soil has the most organic matter and soil life and is depleted of nutrient rich clays and +ve ions, by water passing through. Also known as the zone of alluviation.
< B - subsoil which accumulates iron, clay, aluminium and organic compounds. Also known as the zone of illuviation.
< C - bedrock - parent rock weathered by groundwater.
< R - bedrock - unaltered parent material. A continuous bed of hard rock that cannot be excavated by hand.
The key to managing soil is maintaining a good structure with a high proportion of air filled pores, without restricting water supply.
Soil is made up of different proportions of clay, sand, silt, chalk and peat particles. Its structure is determined by how these particles are held together to form aggregates. The size and shape of these aggregates determine the structural classification of the soil or, ‘soil structure’.
A good soil structure is important to allow air and water into the soil. This is vital for healthy plant growth. It improves drainage and reduces soil erosion caused by excess surface run-off. Without a good structure, soils can suffer from anaerobism, water logging, nutrient lock-up and, ultimately, plants will die! Soils with a good structure can resist compaction and water logging.
Soil Texture
We can also look at soils in terms of texture. For example whether a soil is sandy, clay, silty, loamy or peaty. There are many different textures, as soils are made up of varying mixtures of different particle types. In relation to horticulture this is important, as the different types of soil textures and the overall structure they form, affect a soils cultivation window. A cultivation window is basically the number of days a particular soil is suitable for cultivation. Cultivation windows can be lengthened through the addition of organic matter to improve structure.
Here are all the different types of soil…
Clay
Clay soil - soils with over 25% clay.
These are known as cold soils or heavy soils. They hold a lot of nutrients due to their high Cation Exchange Capacity, but they also hold a lot of water in the tiny spaces between the clay particles, meaning they can be slow to drain and prone to water logging and compaction if they are walked on when wet. They are known as ‘cold’ because they take a long time to warm up in the spring, but can bake in the summer, become hard and crack.
Clay has a high buffering capacity. Although clay can be a difficult soil to manage, with good cultivation and plant choice, the high fertility level of clay soils mean they have a lot of potential for growing plants.
Sand
Sandy soil - Soils with over 85% sand.
A sandy soil has a high proportion of sand particles and not much clay. It is a light soil with a loose structure, making it free-draining and easy to work. The relatively large sand particles warm faster clay soils or soils with lots of organic matter. This means sandy soils have a longer cultivation window. The free-draining nature of sandy soils means they are susceptible to drought. Sand has an unstable structure and nutrients can leach easily from the soil leading to low fertility. They are best dug in spring before the warm weather to reduce water loss. Sandy soils can be very acidic but have a low buffering capacity.
Loam
Loam - soils with around 40% sand 40% silt and 20% clay.
Loam is a mixture of sand, silt and clay in varying proportions, making different types of loams : silty loam, sandy loam, clay loam, silty clay and loam.
Loam soils tend to be more nutrient rich and moisture retentive than sandy soils and have better drainage, porosity and a longer cultivation window than clay soils. It is generally a good soil for growing most types of plant.
Chalk
Chalk - mostly calcium carbonate with a small amount of silt and clay.
Chalky soils tend to be very free draining with a low water holding capacity, meaning they dry out very easily. Despite being fertile, most of the nutrients are unavailable to plants, due to a very high pH . This alkalinity can cause a yellowing of plant leaves due to the inability of plants to take up iron and manganese. Added to this, additional composted materials tend to rot down very quickly and add making them very challenging for gardeners.
Peat
Silt - 80% or more silt with some clay and sand
Silt soils are rarely found in a garden. They are made up of very fine particles that feel slippery when wet. They benefit from a large amount of additional organic matter to improve their structure.
Silty soils have a short cultivation window. It is usually very fertile and holds water well, which can lead to compaction and water logging.
Soil Texture
We can also look at soils in terms of texture. For example, whether a soil is sandy, clay, silty, loamy or peaty. There are many different textures, as soils are made up of varying mixtures of different particle types. In relation to horticulture this is important, as the different types of soil textures and the overall structure they form, affect a soils cultivation window. A cultivation window is basically the number of days a particular soil is suitable for cultivation. Cultivation windows can be lengthened through the addition of organic matter to improve structure.
If you really want to geek our over soils types check out this article article from the University of Michigan!
Water and Air in the Soil
So we know that sand, silt and clay particles are the primary minerals that make up soil. Soil structure affects the movement of water and air through soil, and thus influences its ability to sustain plant life. A soil with a good structure allows air into the soil. Air is essential for preventing a toxic build of the CO2 produced by soil respiration. Increasing levels of CO2 cause a rise in soil acidity, which can in turn affect nutrient uptake by plants.
Water and air are held in and travel through pores, which run in between and through the aggregates.
Macropores - greater than 0.08mm in diameter, these large pores usually run in between aggregates. They are free draining, allowing air and water to pass through them easily. Plant roots can easily penetrate and they provide a vital habitat for soil organisms.
Mesopores - these are medium sized pores which hold most of the water available to plants. They are filled with water and air, giving plant roots access to water for growth, without becoming waterlogged. The more mesopores in a soil, the higher the water holding capacity.
Micropores - are the smallest pores and have a diameter of less than 0.08 mm. Suction by capillary action is required to remove water from micropores and this water is unavailable for uptake by plants.
Ideally, a soil needs enough mesopores to ensure good retention of available water, but sufficient macropores to allow free drainage, root exploration and gaseous exchange. For example:-
Sandy soils (30-40% pore space) - a sandy soil will have a lower available water content than clay soil making it less fertile as nutrients are leached from the soil, and susceptible to drought. However, as it is more free draining, it less prone to water logging and compaction.
Clay soils (50-70% pore space) - although clay has more pore space than sand, it has 3-4 x the number of micropores. Therefore although it holds more available water it doesn’t drain freely making it susceptible to water logging and compaction.
Science Alert!!!!
Skip this next section if you are afraid of science…
Within soil there are chemically charged elements
Anions, which are negatively charged elements,
Cations, which are positively charged elements.
The negatively charged site is on the surface of the soil particle. The larger the surface area, the greater the negative charge! For example: Sandy soils have a lower negative charge than clay or silty soils due to a smaller overall surface area. Soils like clay have particles with a large surface area so hold a larger negative charge.
Now for the clever bit… Many of the nutrients essential for a plants growth are positively charged. They are attracted to negative charge on the surface of the soil particles, just like a magnet. So, soils like clay, with their larger surface area and higher negative charge are more likely to hold more of the nutrients plants need to grow.
A soil’s ability to do this is known as its Cation Exchange Capacity or CEC, and a higher CEC means a more fertile soil and vice versa.
In order to extract the nutrients from the soil, plants make hydrogen cations, which are strongly positively charged, they swap these for other nutrients. However not all nutrients have the same amount of positive charge, some like Aluminium or Iron have a very strong charge, making the harder to exchange. The availability of nutrients in soil depends on the amount of positive charge they have. The stronger the charge, the more the nutrients hold onto the soil and aren’t available to plants.
For more information watch this video
Did you ignore the science alert?
If so you may not understand this!! But don’t worry - you can still find out how to improve your soil.
Soil pH
The availability of nutrients in soil affects the soils pH levels. pH is related to the amount of aluminium and hydrogen present in the soil. A soil with a high Cation Exchange Capacity, such a clay, is more able to rebalance changes in the levels of these two nutrients in the soil to maintain a stable pH. This is known as a buffering capacity.
If you are wanting to change the pH of a clay it will take a large amount of additional materials to have any effect, as the large number of negatively charged sites on the clay particles make them very resistant to pH change. A sandy soil has a lower buffering capacity as it has less negatively charged sites to hold the rebalancing nutrients, making it easier to change the pH.
Knowing the pH of your soil is important when picking you plants for a new garden or if you want to vegetable garden. Don’t panic, it’s cheap and easy to carry out a soil test for the pH of your soil. Blog to follow…
Organic Matter
Organic matter is decaying plant or animal material. In the garden it is most commonly added as green manure, animal manure, compost, peat moss or leaf mould. The process of the decay is called humification and involves the living organisms present in the soil breaking down organic matter into humus. These organisms can be classed as Macro organisms - slugs, snails, earthworms, woodlice, springtails, beetles and nematodes and Microorganisms - bacteria, actinomycetes and saprophytic fungi.
Organic matter must decompose by passage through these organisms, in order for the nutrients present in organic matter to become available to plants. The soil organisms are either macro or micro organisms. The activity of these organisms categorises them into either:-
Primary decomposers -
insects, earthworms and fungi which attack fresh dead organic matter
Secondary decomposers -
bacteria and fungi which live on the waste of the primary decomposers.
Find out more about why composting is so important, not just for your garden but for the planet!!
The humus produced by this process improves all soils. When combined with sand it sticks the sand particles together and when combined with clay it binds to the particles to make them less sticky. This encourages a good crumb structure, colouring the soil darker, increasing soil aggregation and stability, it increases the CEC and buffering capacity, adds nutrients, increases the water holding capacity and increases the soils ability to sequester CO2 from the atmosphere.
Rant Alert!!!
Soil management and carbon and nitrogen
We hear a lot about Carbon in the atmosphere and its effect on the planet. Aaah yes - Global Warming! We need to stop pumping CO2 into the atmosphere in order to save the planet! Well, this may or may not be true, depending on which expert you listen to …. But whatever. Cleaner air can only be a good thing right?
So, we reduce emissions and plant trees to soak up the carbon in the atmosphere. But what about our other major carbon sinks … well yes … there’s our oceans …. And there’s soil!
It is estimated that the ground we are walking on holds 3 times more carbon than the atmosphere! And there is a massive potential for absorbing even more. Unfortunately we know very little about soil and its minerals. It has been suggested that we know more about the surface of Mars than we do about the surface of our own planet.
Globally we need to find a way to balance food production with protecting soil. Scientists estimate that the worlds’ cultivated soil has lost up to 70% of its carbon due to poor management and intensive farming practices. Most of this carbon reacts with oxygen in the air to become CO2 in the atmosphere.
At the moment we don't really know how to best use this huge potential carbon sink beneath us, but ideas around best farming practices such as planting more annual crops and reducing tillage, or ‘No Dig’ systems are being investigated. This importance of soil and its role in the Carbon Cycle must not be underestimated.
What is soil carbon?
Carbon enters the soil via the fixation of carbon in the atmosphere by plants in the form of atmospheric CO2. This is the process we know as photosynthesis. We know that as plants and the animals that eat the plants die, organic matter is formed. This organic matter is broken down by microbial activities and incorporated into soil by macro organisms. Some carbon is oxidised by these soil organisms and released back into the atmosphere. Organic matter that is harder to break down forms long term soil carbon.
The Carbon Cycle
The Continuous movement of carbon between different living organisms on earth and between living organisms and the environment through natural processes like photosynthesis, respiration and decomposition in the soil, and also the burning of fossil fuels.
Cambridge Dictionary
Soil as a Carbon Sink
The Earth's soils contain about 2,500 gigatons of carbon—that's more than three times the amount of carbon in the atmosphere and four times the amount stored in all living plants and animals.
“To Dig or not to Dig? That is the question”
- William Spadesspere (only kidding!!)
When we cultivate soil it becomes exposed to wind and water, increasing erosion. By using minimal tillage or ‘No Dig’ and direct drilling, farmers minimise soil disturbance and therefore reduce erosion. Dig or no dig is a hot topic among gardeners. A lot of research is being carried out to establish the effects of cultivation or ‘tillage’ on the amount of carbon held in soil. It is possible that over time using a minimum tillage method additional carbon is sequestered by the soil due to a build up of organic matter. Further benefits of minimum tillage are:
Improves infiltration rate, water holding capacity, aggregate structure and stability and aeration.
An increase in the nutrient pool
An increase in the process of organic matter decomposition and therefore microbial populations
The soil is protected from wind and water erosion
Nitrogen Cycle
The nitrogen cycle is the biogeochemical cycle by which nitrogen is converted into multiple chemical forms as it circulates among atmosphere, terrestrial, and marine ecosystems.
Wikipedia
The process happens in the following stages:-
Stage 1 - Nitrogen Fixation
Nitrogen makes up 78% of the air. Plants and animals use nitrogen to make the amino acids essential for growth, but they are unable to take it in from the air. Through nitrogen fixation, nitrogen present in the air is turned into a usable form. There are several ways in which nitrogen fixation occurs:-
Free living bacteria called Azotobacter take nitrogen from the air and store it in the form of amino acids. When they die the amino acids break down, leaving ammonia as a waste product which is subsequently turned into nitrates. In this form the nitrogen is able to move through the food chain.
Leguminous plants such as clover, beans and peas can take up nitrogen from the air in soil through Rhizobia, a bacteria living on the nodules of leguminous plant roots, and turn it into nitrates.
Nitrogen is turned into nitrogen oxide by lightning and enters the soil in this form to make nitrates.
Gaseous nitrogen is turned into synthetic fertilizers through the Haber Process. These can then be used to add nitrates to soil artificially.
Stage 2 - Decomposition and Ammonification
Once the nitrogen has been fixed into nitrates in the soil, plants are able to absorb them through their root system. Animals eat these plants, digest and excrete them breaking them down further, or, the animal dies and decays. This dead organic matter decomposes and is consumed by bacteria and other organisms in the process of humification. The nitrogen in the organic matter is converted into ammonium in the soil during this process. In this form the nitrates are again unusable by plants, meaning it must be converted back into a usable form. This happens in stage 3.
Stage 3 - Nitrification and denitrification
The waste product of humification , ammonium, is further broken down by nitrifying bacteria in the process of nitrification. During nitrification ammonia is changed into nitrites in the soil by the bacteria of the nitrosomonas species. These nitrites are in turn converted into nitrates by the bacteria of the nitrobacter species, making it available to plants.
Stage 4 - Denitrification
Denitrification occurs when oxygen levels are too low for aerobic respiration of certain bacteria to occur, resulting in anaerobic respiration of bacteria. This causes nitrates to be converted in their gaseous form and released from the soil back into the atmosphere. Denitrification occurs naturally in all balanced ecosystems and is beneficial when removing nitrates from wastewater. However, it can have a negative effect by removing valuable nitrates from soil and releasing them into the atmosphere as pollutant gases nitrous oxide and nitric oxide.
Phosphorous Cycle
The role of phosphorus in animals and plants
Phosphorus is an essential nutrient for animals and plants. It plays a critical role in cell development and is a key component of molecules that store energy, such as ATP (adenosine triphosphate), DNA and lipids (fats and oils). Insufficient phosphorus in the soil can result in a decreased crop yield.
Stages of the Phosphorus cycle
Weathering causes rocks to release phosphate ions and other minerals into soils and water.
Plants take up phosphate from the soil, and are then, in turn, consumed by animals. When the plant or animal dies and decays, phosphate is returned to the soil.
Within the soil, bacteria break down organic matter into available forms of phosphorus through mineralisation.
When phosphorus in soil is washed into waterways it can, over time, be incorporated into sediments.
Most of our phosphorus is unavailable to plants, being locked up in sediments and rocks. Even much of the phosphorus in soils is not available for uptake by plants. For this reason phosphate fertilisers are used a lot to increase crop yields.
Overfertilization can lead to excess phosphates entering waterways through water runoff and leaching, causing ‘eutrophication’, the excess growth of aquatic plants and algae. This can have disastrous effects on the ecological balance of our waterways - resulting in the depletion of fish and other aquatic animals, and a general deterioration of water quality.